Ralf Knischka (BASF SE Germany), Frank Pirrung (BASF SE Germany), Brandon Achord (BASF Corp. USA), Kyle Flack (BASF Corp. USA), Elena Martinez (BASF SE Germany), Clemens Auschra (BASF SE Germany)
Summary
Star-type surfactants can be seen as the next evolution step from single-chain and gemini surfactants. In this paper the physico-chemical properties of novel silicone-free non-ionic star-type surfactants are compared using dynamic and static surface tension measurements showing clear dependencies of their compositional parameters on the ability to reduce the surface tension as well as the dosage to reach the lowest values. A special focus is placed on their wetting and foam stabilization properties in various water-borne DTM formulations. The results, while formulation dependent where comparable or even improved to high-end gemini surfactants. The dynamic wetting behavior was evaluated using automatic spraying, again showing comparable results versus a gemini surfactant. The performance difference of a standard DTM formulation on the corrosion protection is compared proving the importance of excellent substrate wetting for corrosion protection and the excellent performance of selected star-type surfactants versus the compared high-end gemini benchmarks.
Introduction
The coating of surfaces is done for various reasons including aesthetics and protection. Especially in the case of protection against e.g. corrosion a continuous film with excellent adhesion is of paramount importance. The required adhesion between the coating film and the substrate can only be achieved via an excellent wetting of the substrate by the liquid coating formulation. Having a lower surface tension of the liquid coating relative to the surface tension of the substrate is the most decisive factor for optimum substrate wetting. While it is usually not practical (e.g. by corona treatment) or time consuming (e.g. by applying a primer) to increase the surface tension of the substrate, the surface tension of the coating can be lowered by the addition of surfactants.[1] This lowers the contact angle of the liquid coating on the substrate (see figure 1), and allows the paint to spread and flow more uniformly to form a smooth paint film free of defects.
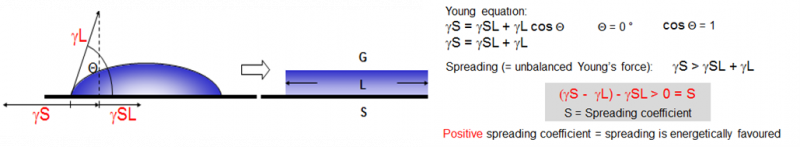
Figure 1: Surface tension and spreading coefficent
Substrate wetting agents or surface-active-agents (surfactants) are therefore indispensable additives in modern paint formulations to achieve homogenous coatings free of defects. This is true for all types of coating systems, including solvent-borne types but especially the case for water-borne coatings with an intrinsic larger difference between the surface energy of the substrate and the liquid coating formulation.
Depending on the substrate (metal versus plastic) and the application speed (spray versus brush) a series of different products can be selected ranging from fluorinated molecules, silicones to hydrocarbon-based surfactants. All these products differ in the degree of surface tension reduction, their dynamic behavior and their foam stabilization tendency.[2] In general, they are composed of a hydrophilic part, or head-group, and a hydrophobic part, or tail-group, within one molecule.
Gemini-type surfactants have been heavily promoted in the market in recent years. They are distinguished by exhibiting a much lower critical aggregation concentration (CAC) than their classical single-chain counterparts leading to a high surface activity. According to the literature this is related to the chemical link of the head groups resulting in two single-chain surfactants being connected.[3] They combine excellent dynamic wetting with low foam stabilization. Gemini type surfactants are available based on either silicone- or hydrocarbon-chemistry and can be also seen as the first step in moving into oligomeric surfactants including star-type products (Figure 2).[4,5]
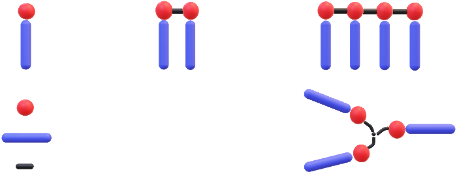
Figure 2: Graphic representation of standard single-chain, gemini and oligomeric surfactants including star-types (from left to bottom right)
For this study we looked into oligomeric hydrocarbon-based surfactants exhibiting star-type branching having a hydrophilic core and hydrophobic shell. We compared their performance versus commercially available gemini and standard single-chain surfactants in CAC, surface energy reduction, dynamic behavior as well as foam stabilization. Due to the unique structure of the star-type surfactants and the in general reported strong hydrophobic interaction of the alkyl groups at higher concentrations we specifically looked into diffusion of these specific novel compounds towards the water/solid interface during coating processes.[7]
We therefore investigated the area of water-borne direct-to-metal (DTM) coatings which is an application trend within the coatings business that is growing in importance.[6] The move from a multilayer application with primer, intermediate coat and topcoat to one single coat is challenging. As of now the multilayer systems are still beneficial when looking into high corrosion classes as C4 and C5, which is true for both solvent- and water-borne systems. A suitable water-borne DTM formulation has to fit from all its ingredients like e.g. the binder, however especially the dynamic behavior is equally important to achieve defect free coatings. This is of paramount importance as only a homogenous film with the right thickness provides consistent protection from corrosion.
Therefore, the effect of the different surfactants on the general wetting behavior and on corrosion protection is compared.
Physical Properties of the Additives
Table 1: Experimental samples with star-type branching
| Core |
Shell |
Sample |
Mn (g/mol) (measured) |
Mn (g/mol) (calculated) |
w% of shell |
| C1 |
Alkyl1 |
Star1150 |
150 |
900 |
50 |
| Alkyl2 |
Star1280 |
300 |
2250 |
80 |
| Star1275 |
400 |
1800 |
75 |
| Star1270 |
350 |
1500 |
70 |
| Star1250 |
250 |
900 |
50 |
| Star1235 |
100 |
700 |
35 |
| Alkyl3 |
Star1350 |
500 |
900 |
50 |
| C2 |
Alkyl1 |
Star2135 |
1000 |
1500 |
35 |
| Star2140 |
2800 |
2300 |
40 |
| Star2145 |
1000 |
1700 |
45 |
Table 2: Comparative samples with gemini and single-chain structure
| Sample |
Mn (g/mol) (measured) |
Mn (g/mol) (calculated) |
Description |
| Gem1 |
n.a. |
226 |
Tetramethyl-decin-diol |
| Stand1 |
550 |
380 |
Alkylalkoxylate |
For this study the series of additives as shown in table 1 and 2 were tested.
In theory different hyper- or star-branched cores are technically available. However, especially in water-borne formulations the hydrolytic stability is a criterion of high importance. We investigated different core technologies being polycarbonate and polyether as well as different chemistries for the hydrophobic shell with ester- and ether linkages. The synthesis of these star-type surfactants is reported elsewhere.[8] The length of the hydrocarbon group is increased from Alkyl 1 to Alkyl 3, being in the range of C8 to C18. A stability test at 50 °C and a pH of 8.5 in a water/butylglycol mixture (19/1) showed, only the combination of a hydrophilic polyether core and a hydrophobic shell linked via ether groups maintained a stable pH. For other structures containing ester groups, the pH rapidly decreased within the test period of 4 weeks indicating the breakdown of the polymer under these conditions. Shifts of the signals of the aged materials in GPC measurements confirmed this assumption.
The molecular weights of the samples were determined by gel permeation chromatography (GPC) in THF, calibrated with polystyrene standards. As expected, due to the difference in hydrodynamic values versus polystyrene, the measured molecular weights for the surfactants with star-type branching differ significantly from the calculated ones (table 1). The values for the star-type surfactants with core C2 show a lower difference between the calculated and the measured values which can be explained by the lower polarity versus core C1, leading to fewer interactions with the GPC column. In order to understand how the structure of these star-type surfactant affects their tendency to move to the interface as well as their aggregation behavior, measurements of static and dynamic surface tensions were executed. The static surface tension was measured in demineralized water at different concentrations utilizing the hanging drop method applying an OCA 100 from Dataphysics.[9]
Graph 1: Static surface tension measurement of additives in demineralized H2O
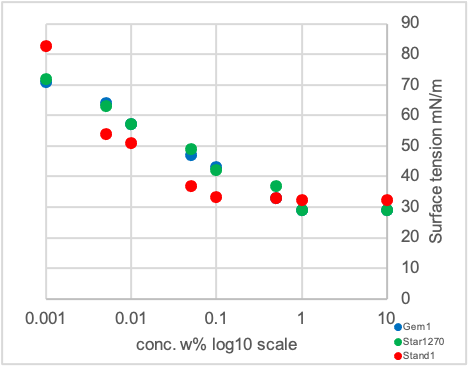
Surface tension measurement at different concentrations allows to assess both the minimal equilibrium surface tension the surfactant can achieve, and the concentration at which any sort for self-aggregation is taking place. Commonly this point is referred to as the critical aggregation concentration (CAC) being the point in the plot where the slope of the graph is changing.
The results very clearly show the differences in aggregation behavior. The Gem1 and Star1270 show a wide concentration range of linear decrease of the surface tension reaching a plateau only above 1 w% (Graph 1, left diagram, blue and green points). According to the literature star-type surfactants already show aggregations at much lower concentrations than the CAC which might explain this broad concentration range of linear decrease.[7] A completely different picture is visible for the standard single-chain surfactant Stand1 with in general a slightly higher minimal equilibrium surface tension of 32 mN/m and an earlier (lower concentration) onset around 0.1 w% addition level to reach this value (Graph 1, left diagram, red points).
In the right diagram of Graph 1 it can be seen, that the structure of star-type surfactants and especially the weight percentage of the hydrophobic shell have a strong impact on the aggregation behavior in solution. Samples with a low amount of shell (Star1235 and 1250, blue and black points) show a lower decrease in the final surface tension versus the samples with a high amount of shell (Star1280, 1275 and 1270, green, orange and red points) with a difference of almost 10 mN/m. In addition, the concentration at which the lowest surface tension is achieved is significantly different and in the order of one magnitude higher for the latter ones. In this series ranging from Star1235 to Star1280 the structure is changing from a less branched to a highly branched one, as the length of the hydrocarbon group is kept constant while the amount of shell is increased, indicating the importance of the star-type structure as well as the polarity for the observed effects.
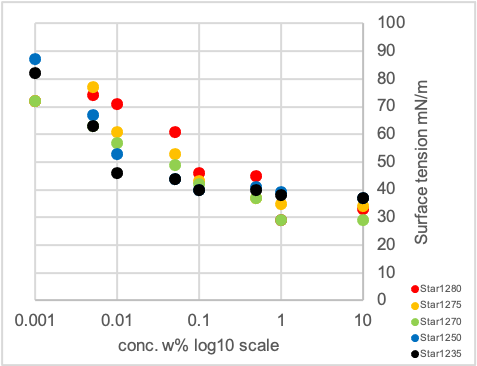
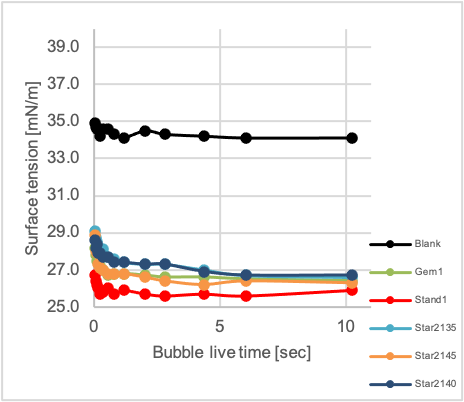
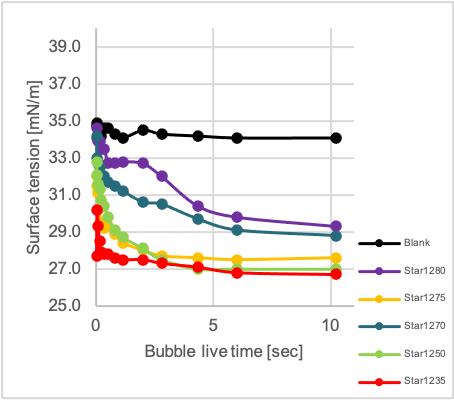
As modern water-borne paints still contain a certain amount of organic solvents, the dynamic surface tension was measured in a mixture of water and butyl glycol, as this is closer to the reality than a measurement in pure water. The dynamic surface tension is a measure how fast an additive can diffuse to the interface liquid/air or liquid/solid and decrease the surface tension. The faster an additive can diffuse the faster the application process can be while still maintaining excellent substrate wetting. A bubble pressure tension meter from SITA was used for this purpose.[10] All products aside from Star1350 were able to reduce the dynamic (i.e. value at 100 ms bubble life time) as well as the static surface tension (i.e. value at 10 s) versus the 19/1 water/butylglycol mixture indicating their high surface activity.
The single-chain product Stand1 is showing the lowest surface tension in the dynamic regime (at a bubble life time of 100 ms), indicating that a low molecular weight is one decisive factor for good dynamic behavior (Graph 2, left diagram). However also the chemical structure is important when comparing Gem1 with Star1150 and Star21xx, all having significantly higher molecular weights. They show comparable dynamic surface tensions which are only slightly above the values for Stand1. Again a very clear dependency of the dynamic behavior on the degree of branching or number of arms respectively could be shown. The amount of hydrophobic alkyl groups is increasing in the series Star1235 to Star1280 (Graph 2, right diagram), the same applies to the dynamic surface tension at 100 ms as well as the static surface tension measured at 10 sec in water/butylglycol 19/1.
Graph 2: Dynamic surface tension measurement of additives in H2O/butylglycol 19/1
Application properties
1) substrate wetting
How these unique physical properties affect the application performance in a coating formulation was tested in two different ways via the wetting behavior.
The first test was based on a contamination of a cold rolled steel panel with a hydrophobic wax (Lusin® Protect G31F). After cleaning of the panels with ethyl acetate, application of the wax and subsequent drying, the additivated coating formulation (based on Joncryl® 8300, colored with a violet pigment paste for better visualization or Acronal® Pro 770, which was white pigmented) was applied to the contaminated steel panel with a wire bare of 75 µm and dried at room temperature. The panel with the cured coating was scanned using a standard office scanner and a jpg image was generated. The surface coverage was detected automatically using a special algorithm.[11]
In general terms, the algorithm is based on 3 steps, which include detecting the coated area in the original image, converting the coated area image into black and white regions, using an automated detection at the appropriate treshhold, and calculating from this image the respective pixel ratio. The higher the surface coverage, the better the surfactant is rated in promoting spreading in this specific coating formula versus the type of substrate.

Figure 3: panels after drying. 3a) draw down blank experiment, 3b) black/white image of 3a, calculated surface coverage 11 %, 3c) draw down with addition of 1.0 w% Star1275 3d) black/white image of 3c, calculated surface coverage 49 %. Formulation based on Joncryl® 8300, violet tinted, substrate wax contaminated cold rolled steel
The blank formulation based on Joncryl 8300 (formulated according to table 4) is showing a very poor wetting behavior with a surface coverage of only 11 % which is clearly improved by surfactant addition (see table 3 column 6 and figure 3). Specifically, the star-type surfactants Star1275 and 1280 are showing an excellent wetting improvement with a surface coverage of 49 and 61 % respectively. The gemini Gem1 and the star-type surfactants based on core C2 Star2xyz are showing a weaker performance in the range of 17-30% coverage.
Looking at the formulation based on Acronal® Pro 770 (see table 3, column 5) we see a comparable behavior with the blank, showing only a moderate substrate wetting on wax contaminated cold rolled steel. As soon as a surfactant is added a significantly improved substrate wetting is observed (aside from sample Star1280 and 1350). The best performance is achieved with the star-type surfactants Star1150, 1250 and 2140 as well as the gemini Gem1.
The second test on wetting behavior is determining the wetting limit of the additivated formulation. This test is focusing on the dynamic application properties during a spraying process. For that purpose, a wedge is applied on a steel panel, starting from low to a high film built. The film thickness where a continuous film is formed defines as the wetting limit. To obtain reproducible results a spray robot is used.[12]


Figure 4: Panel after drying, spray applied. Left image (substrate not wetted, but single droplets visible), middle image (approaching WL, substrate starts to be fully wetted, but some single non-wetted areas visible), right image (substrate completely wetted). Formulation based on Joncryl® 8300, violet-tinted, substrate cold rolled steel
A selection of products was compared (see table 3, column 7) in a formulation based on Joncryl® 8300 (according to table 5). The best performing products were Gem1 and Star1275 followed by Star2145 indicating that the chosen star-type surfactants perform equally in dynamic substrate wetting as the gemini surfactant.
Table 3: Results foam and wetting (spray & draw-down): all properties were rated between 5 (bad) and 1 (good)
| Sample |
Foam |
Foam |
Foam |
Wetting (draw-down) |
Wetting (draw-down) |
Wetting (spray, WL) |
| System |
Acronal® Pro 770 |
Joncryl® 1524 |
Water |
Acronal® Pro 770 |
Joncryl® 8300 |
Joncryl® 8300 |
| Addition level |
0.5 w% |
0.5 w% |
5.0 w% |
0.2 w% |
1.0 w% |
1.0 w% |
| |
|
|
|
Coverage [%], rating |
Coverage [%], rating |
Wetting limit [µm], rating |
| Blank (0%) |
5 |
1 |
n.a. |
70, 4 |
11, 5 |
9, 5 |
| Gem1 |
1 |
2 |
1 |
95, 1 |
17, 4 |
6, 1 |
| Stand1 |
3 |
2 |
5 |
91, 2 |
n.a. |
n.a. |
| Star1150 |
2 |
3 |
3 |
96, 1 |
n.a. |
n.a. |
| Star1280 |
3 |
3 |
1 |
41, 5 |
61, 2 |
8, 5 |
| Star1275 |
2 |
4 |
1 |
90, 2 |
49, 2 |
7, 1 |
| Star1270 |
3 |
2 |
2 |
92, 2 |
45, 3 |
8, 3 |
| Star1250 |
3 |
2 |
3 |
96, 1 |
n.a. |
9, 3 |
| Star1235 |
3 |
3 |
4 |
90, 3 |
n.a. |
n.a. |
| Star1350 |
3 |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
| Star2135 |
1 |
2 |
1 |
92, 2 |
n.a. |
n.a. |
| Star2140 |
1 |
1 |
2 |
95, 1 |
n.a. |
n.a. |
| Star2145 |
1 |
4 |
2 |
91, 2 |
30, 3 |
8, 2 |
2) Foam stabilization
The formation of foam is an important aspect in paint production and application. The time needed to obtain a foam-free formulation adds costs during the paint production. The formation of foam during applicaton (e.g. spraying) leads to visual defects on the coated object that may also lead to performance losses concerning substrate protection. To determine the foam stabilization of the different additives a simplified test formulation was used (see Table 4a, without the defoamers, lines 3 and 9) and 0.5 w% additive (calculated on actives) was added. The formulation was shaken for 30 min using a Scandex® shaker and the foam decay was evaluated via pictures taken after 5, 15, 30, 45 and 60 min. The foam height and its decay was rated between 5 (strong foam) and 1 (no foam).


Figure 5: Foam promotion of a series of additives in an Acronal® Pro 770 based formulation. Left image, blank formulation (rating 5 = severe foaming); right image, addition of 0.5 w% Star2145 (rating 1 = no foam)
As expected, the formulation based on Acronal® Pro 770 containing the surfactant with gemini structure Gem1 is showing significantly less foam compared to the standard (Blank) (see table 3, column 2) but also the formulations containing surfactants with star-type branching (Star21xx) exhibit excellent non-foaming properties on the same level as the formulation containing Gem1. Looking into the products based on core C1 (Star 1xyz) the tests show improved foam destabilization compared to the blank.The formulations based on Star1150 and 1275 show improved properties compared to the single-chain surfactant Stand1.
A different picture is observed when investigating the formulation based on Joncryl® Pro 1524 (see table 4b without the defoamer, line 4). The blank formulation is showing no foaming during the test, and the strongest effect on foam stabilization is seen for the samples Star2145, 1275 and 1280 (see table 3, column 3).
Again, a different picture concerning foam stabilization is obtained when measuring in pure water (see table 3, column 4). A clear differentiation of the performance between star-type surfactants with a high amount of shell like Star1275 and 1280 versus samples with a low amount of shell like Star1235 and 1250 was visible. The more hydrophobic products Star1275 and 1280 show no foaming at all, which is in line with the performance of Gem1. The standard single-chain surfactant Stand1 is showing the strongest foam formation. All star-shaped polymers show a better performance than the single-chain surfactant Stand1.
Table 4a) Formulation details Acronal® Pro 770 (Grind components 1-5 for 30 min at 3000-3500 rpm, rest for 10 h, addition of components 6-13 under stirring)
Table 4b) Formulation details Joncryl® Pro 1524 (Grind components 1-5 for 20 min at 2500 rpm and adjust pH with ammonium hydroxide to > 9, addition of components 6-11 under stirring)
| 4a) Formulation Acronal® Pro 770 |
| # |
Raw material |
Amount [g] |
| 1 |
Water |
8.5 |
| 2 |
Dispex® Ultra PX 4575 |
2.2 |
| 3 |
Foamaster® MO NDW |
0.3 |
| 4 |
DMEA (50% in Water) |
0.1 |
| 5 |
Kronos® 2190 |
19.4 |
| 6 |
Acronal® Pro 770 |
56.4 |
| 7 |
Water |
3.3 |
| 8 |
Butylglykol |
6.5 |
| 9 |
FoamStar® SI 2210 |
0.2 |
| 10 |
Halox® 515 |
2.2 |
| 11 |
DMEA (50% in Water) |
0.2 |
| 12 |
Rheovis® PU 1191 |
0.2 |
| 13 |
Surfactant |
0.5 |
| 4b) Formulation Joncryl® Pro 1524 |
| # |
Raw material |
Amount [g] |
| 1 |
Water |
3.88 |
| 2 |
Dispex® Ultra FA 4416 |
0.08 |
| 3 |
Dispex® CX 4320 |
0.37 |
| 4 |
Foamstar® ST 2446 |
0.19 |
| 5 |
Ti-Pure® R-900 |
15.57 |
| 6 |
Water |
3.88 |
| 7 |
Joncryl® Pro 1524 |
71.83 |
| 8 |
Butyl Cellosolve |
3.57 |
| 9 |
Nazin® FA-179 |
0.1 |
| 10 |
Rheovis® PU 1191 |
0.04 |
| 11 |
Surfactant |
0.5 |
Table 5: Formulation details Joncryl® 8300 (Mix components 1 & 2 and add 10% very slowly to position 3, add component 4 under stirring with max 500 rpm, add the rest of the mixture of 1&2 and components 5-10 under stirring a 2000 rpm for 20 min)
| # |
Raw material |
Amount [g] |
| 1 |
Water |
19.65 |
| 2 |
Butyl glycol |
5.00 |
| 3 |
Luwipal® 066 |
10.80 |
| 4 |
Joncryl® 8300 |
58.20 |
| 5 |
AMP 90 |
0.75 |
| 6 |
Dispex® Ultra PX 4585 |
1.30 |
| 7 |
Foamstar® SI 2250 |
0.20 |
| 8 |
Foamstar® SI 2210 |
0.10 |
| 9 |
Rheovis® HS 1332 |
3.00 |
| 10 |
Surfactant |
1.00 |
3) Corrosion resistance
Further application results have been generated in the area of corrosion resistance, were optimal wetting is a decisive factor. The corrosion resistance of coating systems is divided into 5 classes named C1 to C5, being C5 the highest quality with applications in heavy duty in industrial and coastal environments. In the past this has been a domain of solvent-borne and/or multilayer systems. In the last years direct to metal (DTM) applications have advanced to cover corrosion classes C1 to C3. Specifically, in DTM applications, the wetting behavior is of crucial importance as only one layer is protecting the metal substrate.
The different additives were added in various concentrations to a water-borne coating formulation according to table 4a. The coating was applied at a wet film thickness of 200 µm (leading to 40 µm dry film) using a slit bar on the cold rolled steel panels. The panels were pre-cleaned using ethyl acetate. After application the panel was aged for 2 days at 50 °C and put into the salt spray chamber for 72 h (conditions according to ASTM B117). The corrosion resistance was assessed by the corrosion around the scribe, degree of blistering, rust creepage and dry adhesion.

Figure 6: Panel after corrosion test, coating thickness 40 µm dry, formulation based on Acronal® Pro 770, substrate cold rolled steel, 72 h after salt spray, example with 1 w% Gem1
Most of the samples improve the corrosion protection in the formulation based on Acronal® Pro 770 on the cold rolled steel panel. Samples Stand1, Star1250 and 1275 show no benefits as compared to the blank, and overall have inferior properties, mainly on adhesion properties. Some of the tested star-type surfactants (Star 2135, 2145 and 1235) perform equally well to the benchmark gemini Gem1.
Table 6: Corrosion results, all properties rated with 5 (bad) and 1 (good), addition level 1 w% active
| Sample 1% active |
Corrosion around the scribe |
Degree of blistering |
Rust creepage |
Dry adhesion |
SUM |
| Blank (0%) |
5 |
3 |
2 |
1 |
11 |
| Gem1 |
3 |
2 |
1 |
1 |
7 |
| Stand1 |
5 |
2 |
1 |
5 |
13 |
| Star1150 |
4 |
3 |
1 |
1 |
9 |
| Star1280 |
3 |
4 |
1 |
1 |
9 |
| Star1275 |
4 |
3 |
1 |
5 |
13 |
| Star1270 |
3 |
4 |
1 |
1 |
9 |
| Star1250 |
5 |
3 |
1 |
5 |
14 |
| Star1235 |
4 |
1 |
1 |
1 |
7 |
| Star1350 |
3 |
4 |
1 |
1 |
9 |
| Star2135 |
2 |
2 |
1 |
1 |
7 |
| Star2140 |
3 |
3 |
1 |
3 |
10 |
| Star2145 |
3 |
2 |
1 |
2 |
8 |
Conclusions
Star-type surfactants are a highly interesting class of additives that can provide additional benefits to water-borne coatings in general and specifically DTM coatings. The star-type structure allows the separate tailoring of wetting and foam stabilization behavior. Especially the foam stabilization and the wetting behavior in coating processes can be optimized by the selection of the right core group and the core/shell ratio.
The measured properties in dynamic and static surface tension correlate well with the application results in various DTM systems concerning substrate wetting. The excellent low foaming properties in water did not translate through all test systems, indicating the strong formulation dependency that is known from the coatings industry, leading to the need of a large number of choices of different products in the market space.
Most of the tested star-type surfactants showed a positive effect on the application testing on corrosion protection versus the blank. Some of them show an equal performance to a gemini-type surfactant.
The measurement of the surface tension over a wide concentration range showed the unique aggregation behavior of these star-type surfactant having a hydrophilic core and hydrophobic shell versus standard single-chain products exhibiting a broad concentration range of linear decrease till a flat plateau is reached. It is planned to further investigate this aggregation behavior.
Acknowledgments
We would like to sincerely thank Sascha Bechtel, Lorena di Fede, Miriam Langendörfer, Kai Gigleberger and Emmanuel Haldoupis for their synthesis, testing and other input to this paper.
References
[1] M. Thomson, R. Knischka, , B. Achord, C. Auschra, E. Martinez, American Coating Conference Proceedings, 2014,
[2] S. Oestreich, R. Erhardt, European coatings Journal, 2016, 6, 22
[3] F.M. Menger, Journal of the American Chemical Society, 1991, 113(4), 1451
[4] K. Lehmann, European Coatings Journal, 2004, 5, 32
[5] X.-G. Liu, Colloids and Surfaces A, 2014, 457, 374
[6] S. Silva, European Coatings Online, 2017
[7] Y. Fan, Langmuir, 2018, 34, 11220
[8] F. Pirrung, in preparation
[9] https://www.dataphysics-instruments.com/de/produkte/oca/
[10] https://www.sita-process.com/products/tensiometer/overview/
[11] The “Wetting detector” application was designed to provide an objective quantification of the wetting coverage (coating coverage) of different coating formulations. The application can deal with images of coated panels (substrates) of a specific shape taken at an arbitrary distance and light conditions. The manipulation of the images is carried out using the Python wrapper of OpenCV (https://opencv.org) and the application was built using the pysimplegui python package (https://pysimplegui.readthedocs.io). It is packaged as windows executable using the pyinstaller Python package (https://www.pyinstaller.org/).
[12] http://www.oerter-apl.de/de/